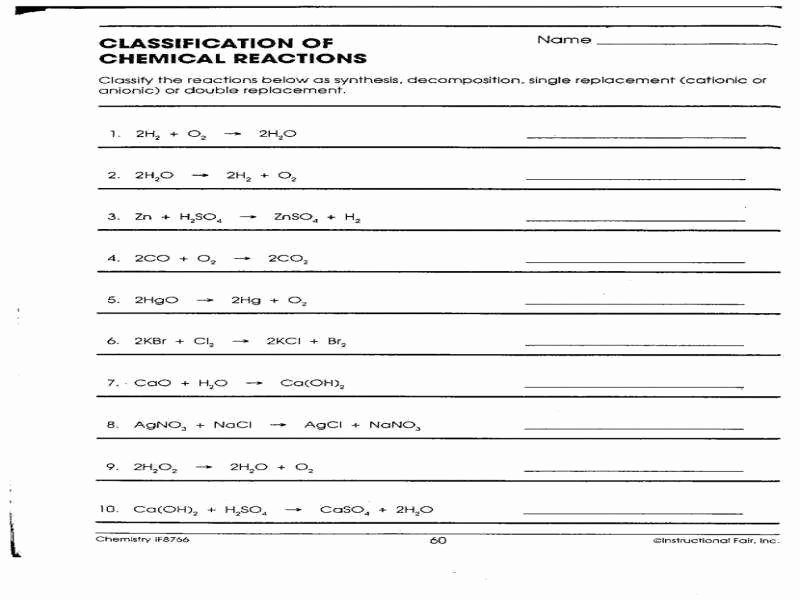
Chemical reactions are an essential part of chemistry. Understanding the different types of chemical reactions can help students grasp the fundamental concepts of chemistry better. This worksheet aims to test the knowledge of students on various types of chemical reactions.
Students will be required to identify and classify different chemical reactions such as synthesis, decomposition, single displacement, double displacement, combustion, and redox reactions. They will also need to balance chemical equations and predict the products of a given reaction.
One of the types of chemical reactions that students will encounter in this worksheet is a synthesis reaction. In a synthesis reaction, two or more reactants combine to form a single product. Students will need to recognize this type of reaction and write the balanced chemical equation for it.
Another type of chemical reaction that students will be tested on is a decomposition reaction. In a decomposition reaction, a single reactant breaks down into two or more products. Students will have to identify this type of reaction and balance the chemical equation accordingly.
Students will also come across single displacement reactions, where one element replaces another in a compound. Double displacement reactions involve the exchange of ions between two compounds to form new products. Combustion reactions involve the rapid combination of a substance with oxygen, often producing heat and light.
Finally, students will encounter redox reactions, where there is a transfer of electrons between reactants. They will need to identify the oxidation and reduction reactions in a given redox reaction and balance the equation accordingly.
By completing this worksheet on types of chemical reactions, students will not only test their understanding of the different types of chemical reactions but also improve their skills in balancing chemical equations and predicting reaction outcomes. This exercise will help solidify their knowledge and prepare them for more advanced chemistry concepts.