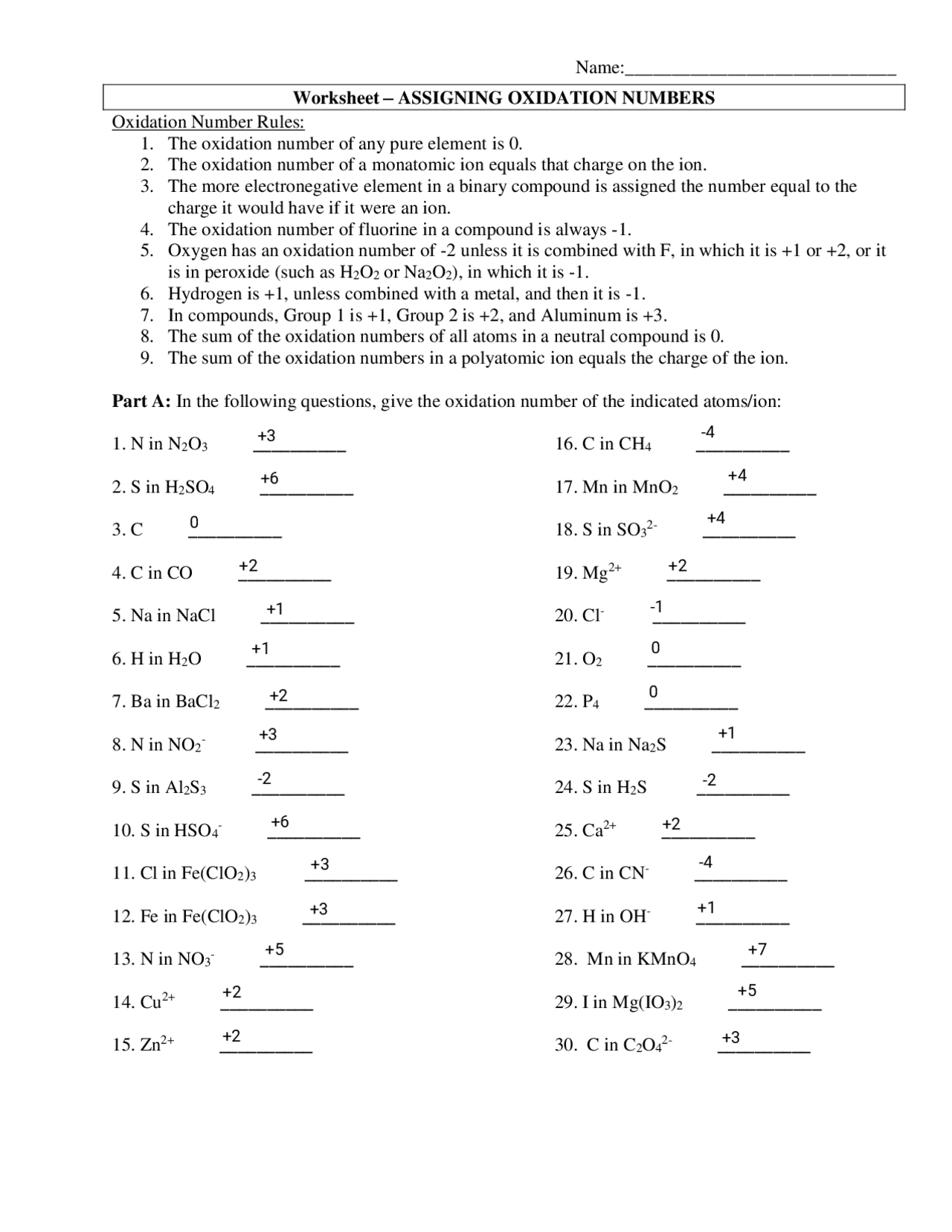
Understanding oxidation numbers is essential in chemistry as they help us determine the charge of an atom in a compound. This information allows us to predict how elements will react with one another and helps us balance chemical equations. In this worksheet, we will practice assigning oxidation numbers to various elements and compounds.
Assigning oxidation numbers involves following a set of rules based on the electronegativity of each element and the overall charge of the compound. By mastering this skill, students can better comprehend redox reactions and the behavior of elements in a chemical reaction.
On the worksheet, students will be presented with a series of chemical compounds and asked to determine the oxidation number of each element. This hands-on practice will reinforce their understanding of oxidation numbers and prepare them for more complex problems in the future.
Students will also have the opportunity to practice balancing redox reactions by using the oxidation numbers they have assigned to each element. This step is crucial in ensuring that the total charge is conserved and that no atoms are lost or gained during the reaction.
By completing this worksheet, students will develop a strong foundation in assigning oxidation numbers and balancing redox reactions. These skills are fundamental in chemistry and will serve as building blocks for more advanced topics in the field.
Overall, this worksheet on oxidation numbers is designed to challenge students and enhance their understanding of chemical reactions. By practicing assigning oxidation numbers and balancing redox reactions, students will build confidence in their abilities and be better prepared for future chemistry courses.